Robotic Capsule Endoscopy by RoboMed

- Smoking
- Eating fast food
- Drinking alcohol
- Aging
- No exercise
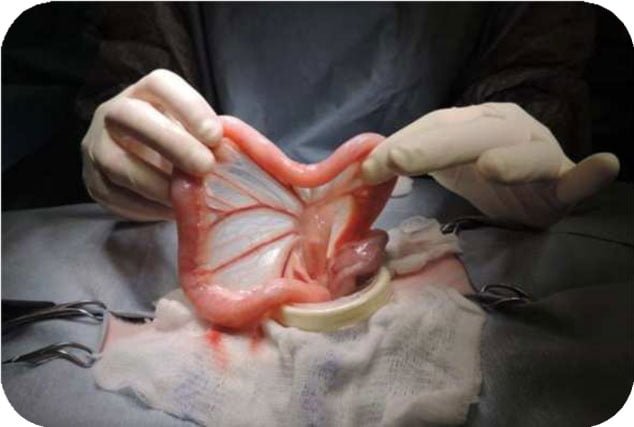
Revolutionizing Diagnostics: Our Robotic Capsule Solution
Enter the future of medical diagnostics with RoboMed’s innovative Robotic Capsule. Specializing in endoscopy and biopsy sampling of the small intestine, our advanced technology ensures precision, comfort, and unparalleled insights. Say goodbye to invasive procedures and hello to a new era of seamless, patient-friendly diagnostics. Your health, reimagined.
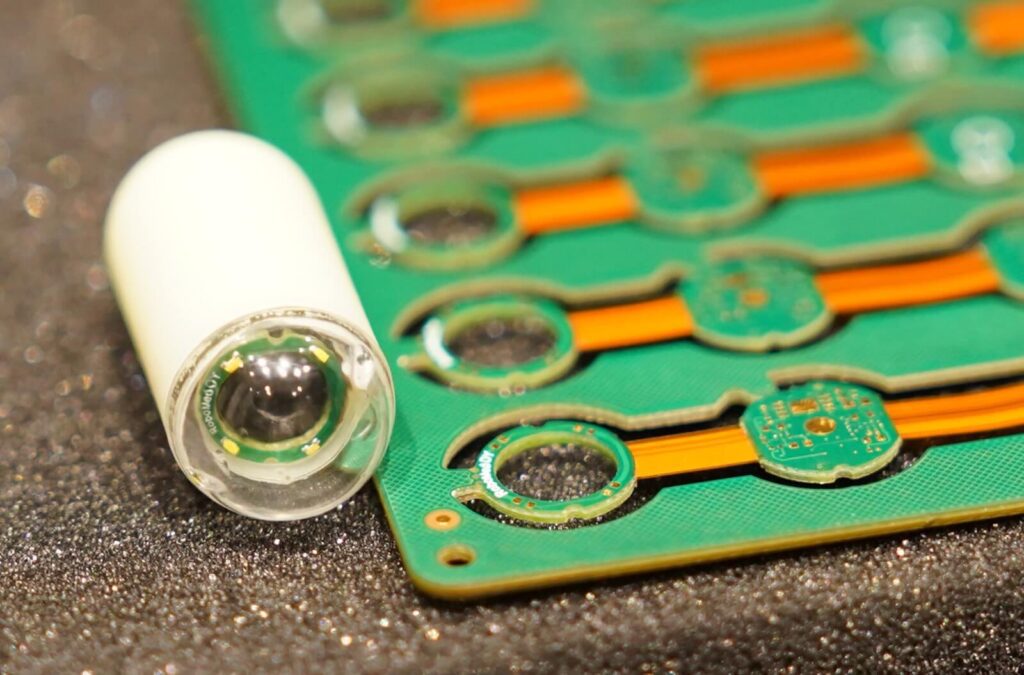
How it works ?
Endoscopy and Biopsy simultaneously
Capsule is carrying Biopsy Micro Needles
Take out sample for pathology and examinations
Innovative Diagnostics, Simplified
Real-Time Biopsy: Immediate insights at your fingertips.
App-Supported & IoT-Based: Seamless integration for on-the-go analysis.
Quick, Fast & Remote: Swift access, anytime, anywhere.
Smart Interface: Intuitive smartphone and computer applications for real-time data.
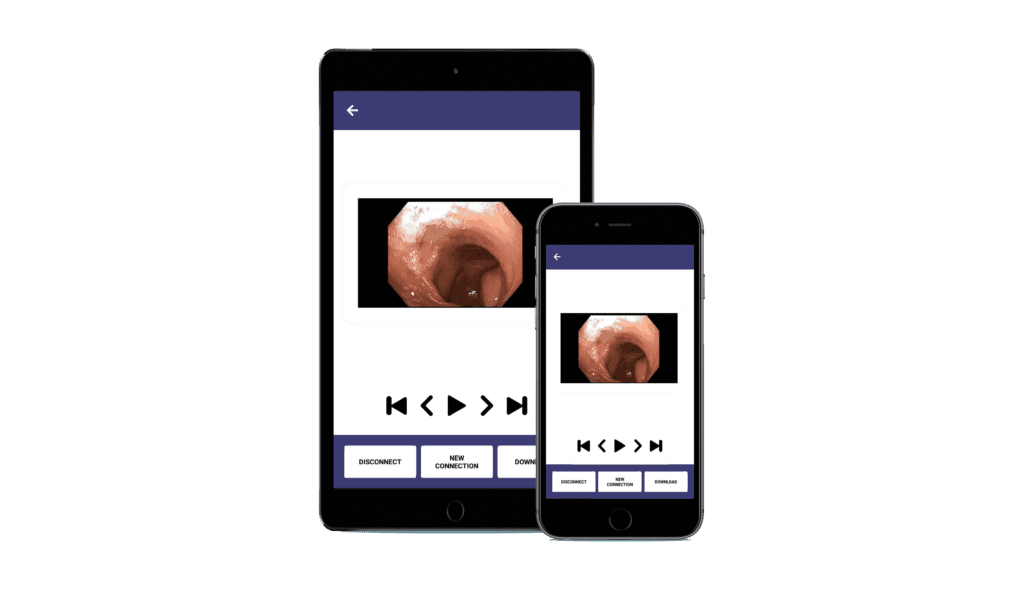
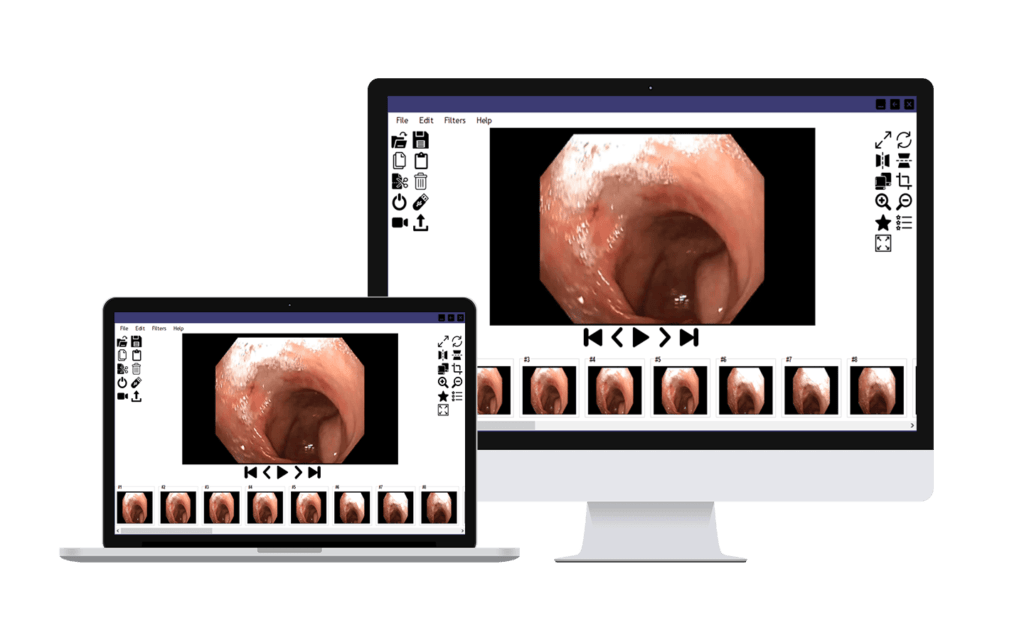
RoboMed products for better and easier medical services
Our Partnership













